Abstract
INTRODUCTION In allogeneic hematopoietic stem cell transplantation (HSCT) for acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL), intensified myeloablative conditioning regimens have been studied to reduce the frequency of post-HSCT relapse. Our group previously analyzed leukemia cases using the Japanese registry database, and found that addition of high-dose cytarabine (AraC) showed superior overall survival (OS) compared with the conventional myeloablative regimen (cyclophosphamide plus total-body irradiation [CY/TBI]) with decreased post-HSCT relapse and the same level of TRM in cord blood transplantation (CBT). Moreover, the addition of etoposide (VP16) to CY/TBI improved the outcome in bone marrow (BM) or peripheral blood stem cell (PBSC) transplants in patients with high-risk ALL.
These results had certain limitations, due to heterogenous donor sources, variable conditioning regimens and time-frame bias (older vs. more recent years of HSCT). These limitations led us to validate the previous results using a large international cohort from the Center for International Blood and Marrow Transplant Research (CIBMTR) in the United States (U.S.) and the Japanese Society for Transplantation and Cellular Therapy (JSTCT) to compare the merit/demerit of these regimens between different cohorts.
PATIENTS and METHODS
We included adult patients (18 years or older at transplantation) who underwent first myeloablative allogeneic HSCT (BM or PBSC) for acute leukemia in remission (CR1 or 2) between 2010 and 2018, either in Japan or the U.S. using conditioning regimens of CY/TBI, CY/TBI+AraC, or CY/TBI+VP16 without any ex vivo T-cell depletion or post-transplant CY. Two datasets from the U.S. and Japan were merged for analysis.
The primary endpoint was the overall survival (OS), and the secondary endpoints included disease-free survival (DFS), therapy-related mortality (TRM), disease relapse/progression, acute and chronic graft-versus-host disease (GVHD), cumulative incidence of infections (bacterial and viral) and veno-occlusive disease/ sinusoidal obstruction syndrome (VOD/SOS) by day 100 post-HSCT.
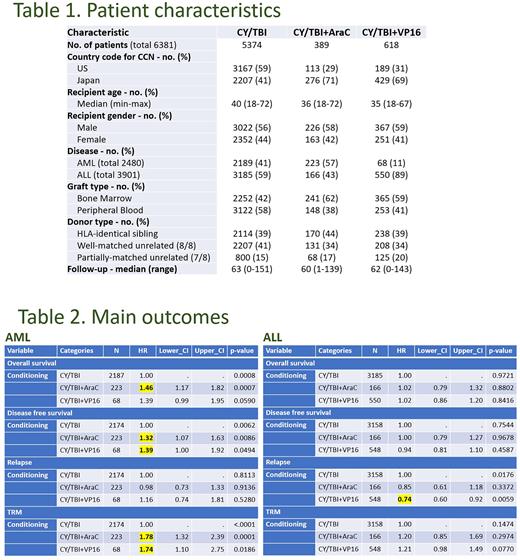
RESULTS In total, 6381 patients were included in this study (3469 from the U.S., and 2912 from Japan) (Table 1). CY/TBI was most commonly used (N = 5374, 84.2%), while CY/TBI+AraC was used in 389 patients and CY/TBI+VP16 in 618 patients. Median age of the patients was 40, 36, and 35 years in CY/TBI, CY/TBI+AraC, and CY/TBI+VP16, respectively. The majority of the patients (N = 5007, 78.4%) were transplanted at CR1. The most frequently used donor sources included HLA-matched unrelated donor (N = 2546, 39.8%), followed by HLA-matched sibling (N = 2522, 39.5%).
The AML cohort (N = 2480, 38.8%) indicated that OS was poorer in CY/TBI+AraC (hazard ratio [HR] 1.46, p < 0.001) and CY/TBI+VP16 (HR 1.39, p = 0.059) compared to CY/TBI. DFS was significantly inferior in these intensified regimens (HR 1.32 and 1.39, p = 0.008 and 0.049, respectively). Relapse was not suppressed (HR 0.98 and 1.16, p = 0.913 and 0.528), while TRM was significantly higher (HR 1.78 and 1.74, p < 0.001 and 0.018, respectively) (Table 2). Incidence of GVHD and infections were comparable among the regimens. Interactions regarding the country were not observed.
In the ALL cohort (N = 3901, 61.2%), OS was comparable among the regimens (HR 1.02, p = 0.880 in CY/TBI+AraC and HR 1.02, p = 0.841 in CY/TBI+VP16, compared to CY/TBI). DFS was also comparable. The merit of intensified regimen was observed in relapse; relapse was significantly suppressed in CY/TBI+VP16 (HR 0.74, p = 0.005), while TRM was higher with borderline significance (HR 1.21, p = 0.077) (Table 2). Other outcomes including GVHD and infections showed no clear differences, according to the conditioning regimens. No interactions were observed regarding the country.
DISCUSSION Our analyses using international datasets provided new insights regarding intensified myeloablative conditioning regimens. In AML, adding AraC to CY/TBI resulted in increased TRM without reducing relapse; thus, it had an adverse effect on OS. Conversely, in ALL adding VP16 or AraC to CY/TBI did not affect survival, but addition of VP16 resulted in lowering the risk of relapse. These trends were comparable between the Japan and U.S. cohorts. Therefore, intensified myeloablative regimens are not recommended in BM or PBSC HSCT, except for CY/TBI+VP16 in ALL.
Disclosures
Atsuta:Meiji Seika Pharma Co, Ltd.: Honoraria; Mochida Pharmaceutical Co., Ltd.: Honoraria; Astellas Pharma Inc.: Honoraria; AbbVie GK: Honoraria; Kyowa Kirin Co., Ltd: Honoraria; Novartis Pharma KK: Honoraria. Wood:Pfizer: Research Funding; Genentech: Research Funding; Teladoc: Consultancy. Kanda:Mundipharma Pharmaceuticals: Research Funding. Kanda:asclepia: Honoraria; ASAHI KASEI PHARMA CORPORATION: Honoraria; Janssen Pharmaceutical K.K.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb Co: Honoraria; Novartis Pharma K.K.: Honoraria, Membership on an entity's Board of Directors or advisory committees; TEIJIN PHARMA LIMITED.: Honoraria; CHUGAI PHARMACEUTICAL Co., Ltd.: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; DAIICHI SANKYO Co., Ltd.: Honoraria, Membership on an entity's Board of Directors or advisory committees; SymBio Pharmaceuticals, Ltd.: Membership on an entity's Board of Directors or advisory committees; Sanofi K.K.: Honoraria; Kyowa Kirin Co., Ltd.: Honoraria; Megakaryon Co: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceutical K.K.: Honoraria; Ono Pharma Inc.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; Amgen Pharma Inc.: Honoraria; AbbVie Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas Pharma Inc.: Consultancy, Honoraria; MSD K.K.: Honoraria; CSL Behring K.K.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; NIPPON KAYAKU CO.,LTD.: Honoraria; Nippon Shinyaku Co., Ltd.: Honoraria; Eisai: Research Funding. Teshima:Fuji Pharma: Research Funding; NIPPON SHINYAKU: Research Funding; TEIJIN PHARMA: Research Funding; Astellas: Research Funding; Chugai: Research Funding; Bristol-Myers Squibb: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; Merck Sharp & Dohme: Honoraria, Membership on an entity's Board of Directors or advisory committees; Luca Science Inc.: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Manuscript preparation, Research Funding; Janssen: Other: Manuscript preparation; Sanofi: Research Funding; Kyowa Kirin: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal